What is the ionic radius of lead?
202 pm Lead/Van der Waals radius
What element has the ionic radius?
6: Definition of Ionic Radius. (a) The internuclear distance is apportioned between adjacent cations (positively charged ions) and anions (negatively charged ions) in the ionic structure, as shown here for Na+ and Cl− in sodium chloride….Ionic Radii and Isoelectronic Series.
| Ion | Radius (pm) | Atomic Number |
|---|---|---|
| Al3+ | 57 | 13 |
What’s the atomic radius of lead?
180 pm Lead/Atomic radius
What elements have the highest ionic radius?
As can be seen in the figures below, the atomic radius increases from top to bottom in a group, and decreases from left to right across a period. Thus, helium is the smallest element, and francium is the largest.
What is ionic radius?
225 pm Tin/Van der Waals radius
What is ionic radius in chemistry?
Ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. Although neither atoms nor ions have sharp boundaries, they are treated as if they were hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice.
How do you find the atomic radius of an element?
The radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance.
How do you determine the largest ionic radius?
In general:
- Ionic radius increases as you move from top to bottom on the periodic table.
- Ionic radius decreases as you move across the periodic table, from left to right.
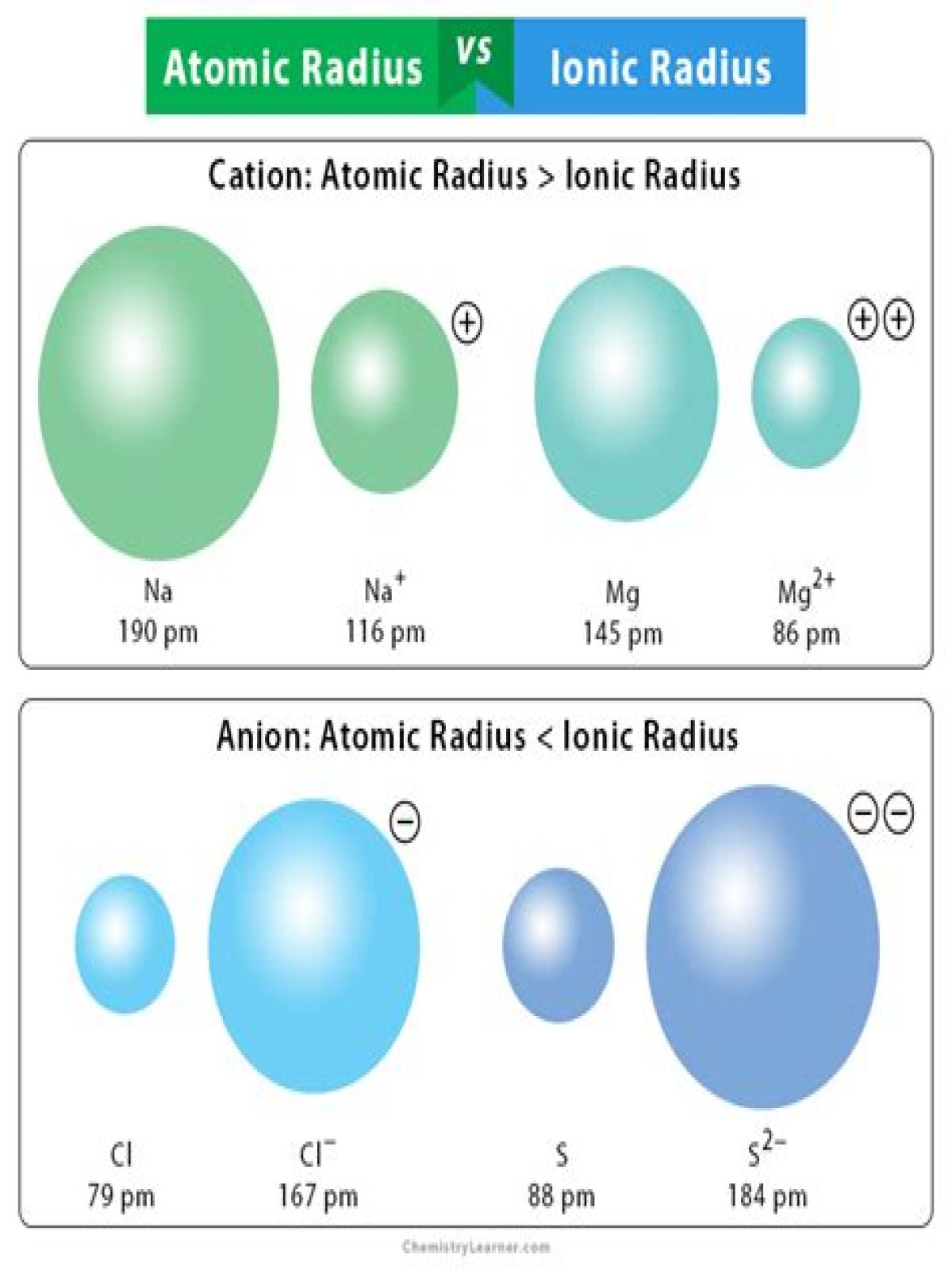
What is the difference between ionic radius and atomic radius?
Atomic is the distance away from the nucleus. Atomic radius increases going from top to bottom and decreases going across the periodic table. Ionic radius is the distance away from the central atom. Ionic radius increases going from top to bottom and decreases across the periodic table.